NANDO – a key base for compliant trade in the EU on the example of the segment of medical devices
Publication date: April 25, 2024
The NANDO (New Approach Notified and Designated Organizations) database is an indispensable tool for every sponsor or investor who wants to legally introduce their products to the European Union market.
NANDO constitutes a list of notified bodies authorized to issue documents confirming compliance with EU regulations. Notified unit is an organization appointed by EU Member State (or other countries under specific agreements) to assess the compliance of certain products with the regulations before placing them on the market. As the name NANDO suggests, it refers to all organizations in the European Union notified and designated by the European Union under the political concept called “New Approach” and “New Approach Framework”. This covers a wide range of products, including toys, personal protective equipment and diagnostic devices.
These entities are authorized to perform tasks related to the conformity assessment procedures set out in the applicable legislation. Access to the NANDO database is possible via the European Commission website: https://ec.europa.eu/growth/tools-databases/nando/
The information contained in the NANDO database is extremely valuable for sponsors and investors because it allows them to:
- Finding the right notified body: Choosing the right notified body is crucial to obtaining a document of compliance with EU regulations. The NANDO database makes this process easier by providing access to a comprehensive list of notified bodies in every EU country.
- Verification of the legality of the notified body: Before you start working with a notified body, it is important to make sure that it is actually authorized to issue documents of conformity. The NANDO database allows you to check the status of a notified body and the scope of its accreditation.
- Obtaining current information: The NANDO database is regularly updated with new information on notified bodies and EU regulations on medical devices. Thanks to this, sponsors and investors can be sure that they are using the latest data.
How to use the NANDO database?
Using the NANDO database is simple and intuitive. On the NANDO website you can find a list of notified authorities by country and specifications for the various EU directives and regulations. After selecting the appropriate country and product category, the user receives a list of authorities competent in a given area, along with their contact details.
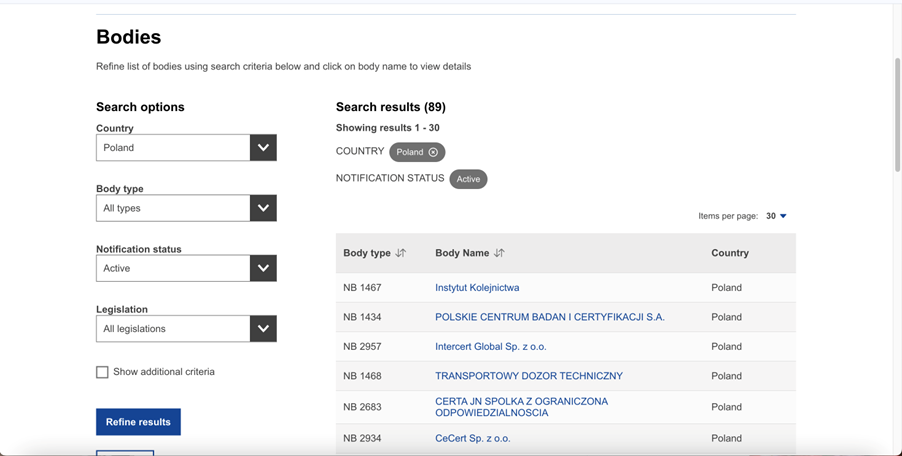
The picture below shows several Polish units in the NANDO database, after narrowing the search to the country in which we are looking for a unit:
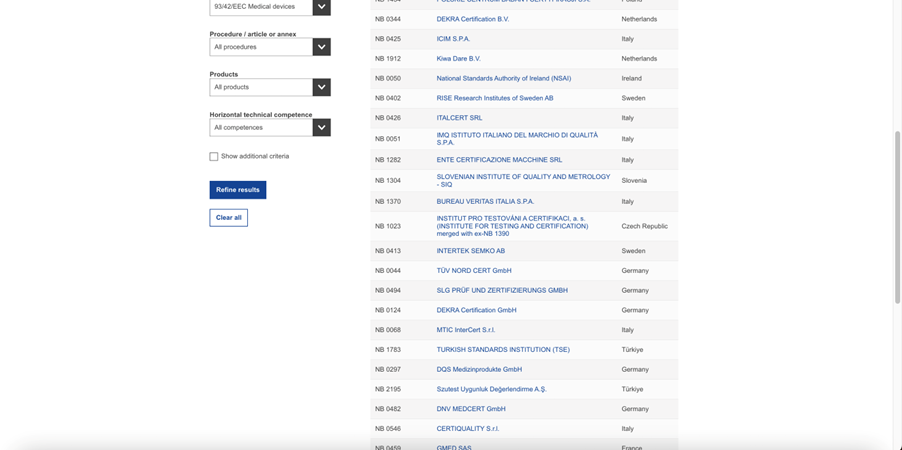
The photo below presents the units after narrowing the search to units related to medical devices in relation to Directive 93/42/EEC:
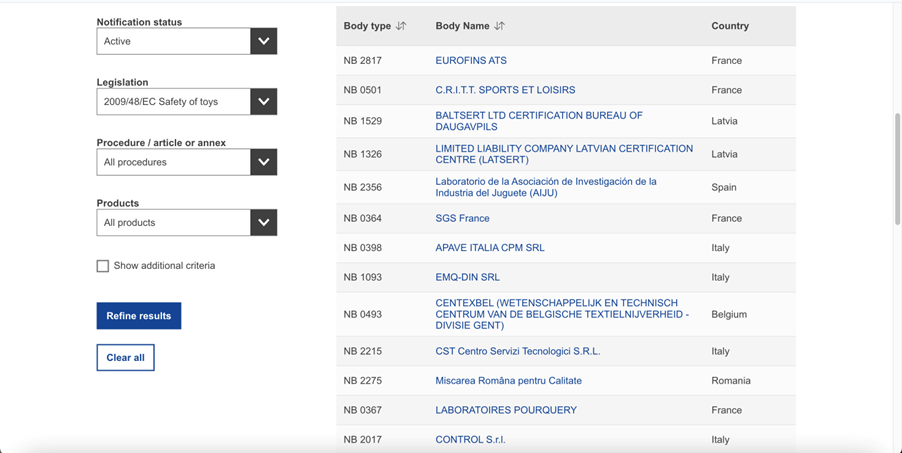
In the photo above, the unit is narrowed down to the units relating to toy safety in relation to Directive 2009/48/EC.
The process of qualification and accreditation of institutions in the NANDO database on the example of medical devices
Legal basis: Article 42 of Regulation 2017/745
Article 42 of Regulation 2017/745 provides that notified bodies may be authorized by Member States to assess the conformity of medical devices with the requirements set out in the Regulation. This means that institutions can be selected and qualified as notified bodies authorized to carry out conformity assessments of medical devices.
Institutional qualification process
Competence verification
Pursuant to Art. 42, notified bodies must have appropriate competences and technical resources to carry out conformity assessments. The qualification process could include an audit and assessment by the relevant national authorities and the European Medicines Agency (EMA) to confirm that the required standards are met.
Accreditation by national authorities
After verification of competence, the unit had to be officially accredited by the relevant national authorities, such as the Ministry of Health. This accreditation confirms that the institution meets all the requirements specified in Art. 42 of the Regulation 2017/745 and is authorized to carry out conformity assessment of medical devices.
Subscription to the NANDO database
After obtaining accreditation, the unit is entered into the NANDO database as an official body notified in Poland. Thanks to this, this institution would become an entity authorized to assess the compliance of medical devices with EU regulations.
The process of verifying the compliance of a medical device by an entity
Step 1: Selecting the country and product category in the NANDO database
By using the NANDO database, sponsors and investors can select Poland as the country of product introduction and the appropriate product category (e.g. medical devices).
Step 2: View the list of notified authorities
In the NANDO database you will find a list of authorities in Poland, along with contact details.
Step 3: Contact the selected entity
A sponsor or investor wishing to introduce a medical device to the EU market should contact the entity of its choice to assess the compliance of the device with the requirements specified in the Regulation 2017/745.
The NANDO (New Approach Notified and Designated Organizations) database is an extremely comprehensive tool that does not only apply to medical devices. It covers a wide range of products and sectors that have specific compliance and security requirements. Below there are presented some examples of products and sectors to which NANDO database can be used:
- Electrical and electronic equipment (Directive 2014/35/EU)
- Building materials (EU Regulation No. 305/2011)
- Personal protective equipment (EU Regulation 2016/425)
- Plant protection products (EU Regulation No. 1107/2009)
Each of these sectors has its own specific regulatory requirements that must be met before a product can be placed on the EU market. Therefore, the NANDO database is an extremely valuable tool for manufacturers, importers and distributors operating in these sectors, helping them to identify the appropriate notified bodies and comply with the required compliance standards.
The NANDO database is not only a tool for searching for notified bodies. It is also a valuable source of information about current EU regulations regarding medical devices.
Regular verification of this information allows sponsors and investors to:
- Stay up to date with the latest changes in EU regulations: EU rules on medical devices are regularly updated. Access to the latest information allows sponsors and investors to adapt their activities to the applicable requirements.
- Avoid errors : Failure to comply with EU regulations can result in serious consequences, such as fines, product withdrawal from the market, or even a ban on placing products on the EU market.
- Increase competitiveness: Knowledge of EU regulations can help sponsors and investors develop safe and effective medical devices that meet the requirements of patients and healthcare professionals.
Bibliography:
https://eur-lex.europa.eu/legal-content/PL/TXT/?uri=CELEX%3A32017R0745
https://webgate.ec.europa.eu/single-market-compliance-space/#/notified-bodies/by-country
https://health.ec.europa.eu/medical-devices-topics-interest/notified-bodies_pl
https://single-market-economy.ec.europa.eu/single-market/goods/building-blocks/notified-bodies_en
