Does your company deal with microplastics? Beware of new regulations!
Publication date: January 20, 2026
Analysis of Commission Regulation (EU) No 2023/2055
EU Commission Regulation 2023/2055 expands Annex XVII of Regulation (EC) 1907/2006 (REACH) by adding entry 78. Annex XVII consists of a list of organic substances to varying degrees. Regulation 2023/2055 was introduced to restrict the use of synthetic polymer microparticles, often referred to as microplastics. However, it should be noted that the definition in the Regulation is broader than the commonly understood definition of microplastics. This initiative aims to reduce their release into the environment, as it is estimated that currently 42,000 tons of intentionally added microplastics enter the environment each year. The Regulation entered into force in 2023, but many provisions will not enter into force until the coming years.
This article aims to analyze the Regulation and facilitate its understanding and practical implications. The key point is the left-hand column of Item 78, which defines synthetic polymer microparticles. These are solid polymers that meet both of the following criteria:
1/ are contained in particles and constitute at least 1% m/m or form an uninterrupted surface of this particle (the concentration will not be important here);
2/ at least 1%m/m of these particles meet:
a/ all particle dimensions are less than or equal to 5 mm (there is no lower limit, although 0.1 µm is assumed, as this is the smallest size that can currently be analyzed, but if the technology changes, the limit will also change);
b/ the length of the particles is equal to or less than 15 mm and the length to diameter ratio is greater than 3.
However, when analyzing the definition, exceptions are equally important, which include polymers formed by natural polymerization processes (regardless of extraction, not chemically modified); polymers that are degradable in accordance with Appendix 15 (which specifies the rules for confirming degradability); polymers that have a solubility greater than 2 g/l in accordance with Appendix 16 (which specifies the rules for confirming solubility), and polymers that do not contain carbon in their chemical structure. If a polymer does not meet the above definition, it will not be covered by the rules of entry 78. This definition requires a case-by-case analysis of whether a given polymer will be considered a microparticle; the EU Commission will not prepare a list specifying precisely which polymers are excluded.
The right-hand column of item 78 describes the restrictions that apply to the polymers identified above. Paragraph 1 prohibits the marketing of polymers as substances on their own (pure polymers) or, where synthetic polymer microparticles are present in a product to impart desired properties, in mixtures at concentrations equal to or greater than 0.01% w/w (this limit was chosen because below this concentration, the addition of polymers does not affect the product’s characteristics). Therefore, this is not a ban on the use of microparticles, but only on their marketing. Furthermore, it does not apply to products in which polymers are an integral part. If the presence of polymers is not intentional, then the product will not be covered by the marketing ban. Paragraph 2 provides more definitions to help understand the above definitions. Paragraph 3 specifies the methods for measuring polymer concentration for the purposes of paragraph 1.
Paragraphs 4 and 5 outline exceptions to the prohibition imposed by paragraph 1. Paragraph 4 covers substances used in industrial plants, medicinal and veterinary products, fertilizer products, food, in vitro diagnostic devices, other food (not regulated by the same regulation), and feed. All exceptions are governed by other EU regulations, which typically specify rules for waste disposal so that they do not pollute the environment. However, the first exception (substances used in industrial plants) must, from October 17, 2025, provide the following information: instructions for use and disposal that prevent the release of microparticles into the environment; a corresponding statement; information on the amount or concentration of microparticles; and general information about microparticles in the product. If the process for proper particle disposal is so complex that the user will not use it, then the exception does not apply. Similar restrictions have been imposed on the exception for in vitro diagnostic devices , which, from October 17, 2026, must provide information on how to use and dispose of microparticles to prevent them from entering the environment. The exception for the first category of food is also covered by this obligation from October 17, 2025: the exception for food 1 in 4 and the exceptions in point 5 must do the same. The rules specifying the requirements for instructions are contained in paragraph 10, and their proper application will be assessed by member states.
Paragraph 5 includes microparticles that are so “separated” that they do not escape into the environment, microparticles whose physical properties have changed so much during use that they no longer fall under the definition (e.g., if they absorb water and thus permanently change their volume), and microparticles that are permanently enclosed in a solid form (will not escape into the environment). These exceptions are also subject to the obligation to provide appropriate information on how to use and dispose of microparticles from October 17, 2025.
Paragraph 6 outlines the exceptions to the ban, which come into effect on October 17, 2023 (as defined in paragraph 16), for which a transition period has been granted, but businesses are encouraged to comply with the regulations before this period expires. This period is presented in the timeline below, with further explanations below.
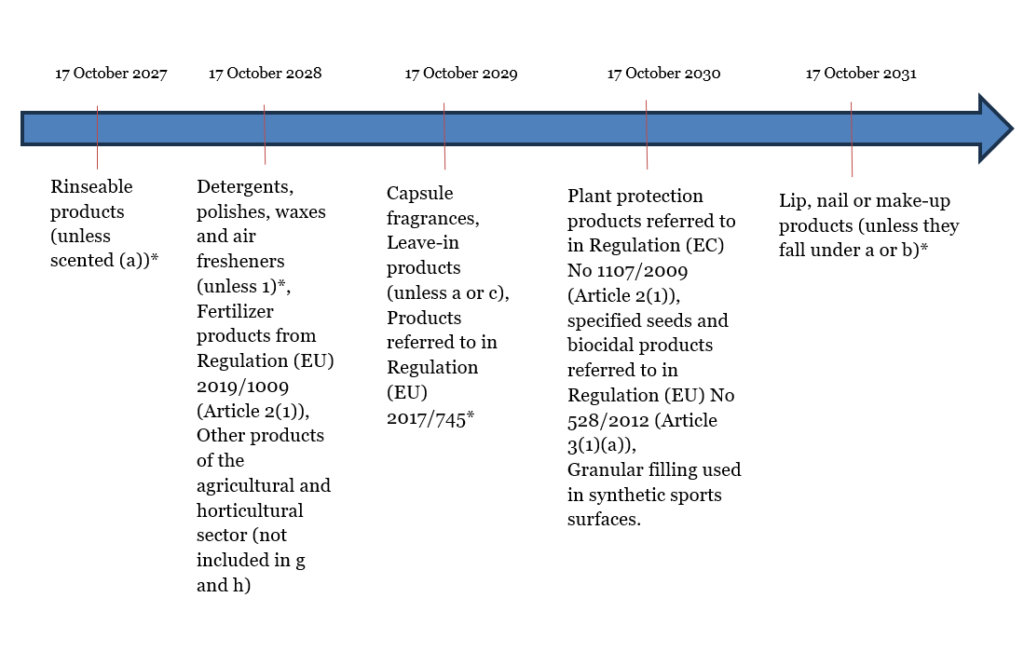
*Microplastics are not covered by the transition period and are therefore an exception to these rules.
Additionally, the products specified in point c from 17 October 2031 (transition period applies) until the sales ban is in force will have to have labels specifying that they contain microplastics.
Paragraph 11 further specifies the information that polymer manufacturers and downstream users (defined in Article 3(13) of REACH) will have to submit to the European Chemicals Agency (ECHA) from 2026 or 2027, depending on the purpose for which the polymers are used. Paragraph 12 imposes similar obligations from 2027; information collected by the Agency will then be made available to Member States in accordance with paragraph 13. In addition, manufacturers, importers, and industrial downstream users are required to make certain information on polymers available to competent authorities within a specified timeframe upon request (paragraph 14). If manufacturers, importers, or downstream users rely on the exceptions in paragraph 5, they are required to provide information confirming such classification, consistent with Appendices 15 and 16, without delay upon request by the competent authorities (paragraph 15).
At the time of writing, the European Commission had published three guides clarifying the classification of polymers included in entry 78. Additional guidance was provided in documents published by the European Chemicals Agency. Unfortunately, most of these are currently only available in English.
In practice, the regulation means significant changes for the cosmetics sector, as this product category cannot benefit from exemptions. Many ingredients, such as exfoliants or glitter, will have to be replaced with biodegradable alternatives in a short period. This is not surprising, however, as some cosmetics industries began restricting the use of plastic microbeads in products such as shower gels and scrubs as early as 2015. One reason for the long transition period is that some substances, such as emulsifiers and thickeners, still lack biodegradable alternatives, or they are not yet widely available. Nevertheless, this is a direction in which many companies will now be forced to develop their research to be able to phase out microbeads by the time the restrictions come into force.
Of course, the cosmetics industry isn’t the only one affected by the ban, but it’s widely seen as one of the most affected. Many other companies are exempt from the use of microparticles to add specific characteristics. It’s worth mentioning that polishes and detergents will also need to spend time developing new solutions. However, the exclusion from Item 78 of products in which microparticles cannot escape or are not intentionally inserted further limits the products that will be subject to the ban.
Sources:
Commission Regulation (EU) 2023/2055 of 25 September 2023 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards synthetic polymer microparticles (Text with EEA relevance)
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC (Text with EEA relevance)
European Commission, ‘REACH restriction of synthetic polymer microparticles (Entry 78 of Annex XVII REACH, as introduced by Commission Regulation (EU) 2023/2055) – Explanatory Guide – Version 1.1’ (European Union 2025)
SGS, ‘The EU has issued regulations regulating microplastics under Annex XVII of the REACH Regulation. The provisions of the new law will be implemented in stages, starting from October 17, 2023’ (SGS, December 5, 2023) <https://www.sgs.com/pl-pl/aktualnosci/2023/12/nowe-przepisy-regulujace-mikroplastik> accessed December 15, 2025
